Steel is an alloy consisting of \(\ce{Fe}\) with a small amount of \(\ce{C}\). Elemental \(\ce{Cr}\) can be added to steel to make the steel less likely to rust; \(\ce{Cr}\) atoms react with oxygen in the air to form a nonreactive layer of chromium oxide on the surface of the steel, preventing the oxidation of underlying \(\ce{Fe}\) atoms. A sample of steel-chromium alloy contains 15 percent \(\ce{Cr}\) by mass. Which of the following diagrams best shows a particle-level view of a surface section and an interior section of the alloy represented below at the left? (The atomic radii of the atoms involved are given in the table below at the right.)
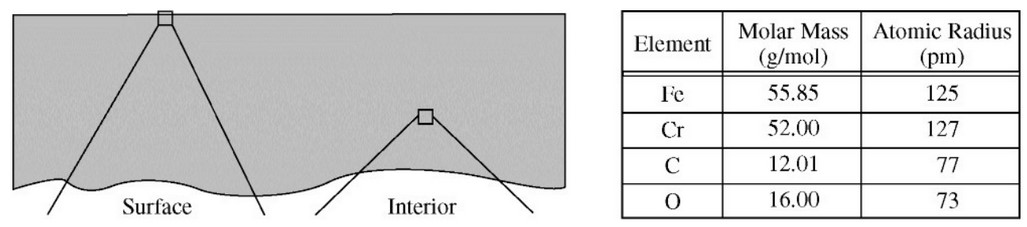
For the answer choices, the image on the left represents the surface section. The image on the right represents the interior section.