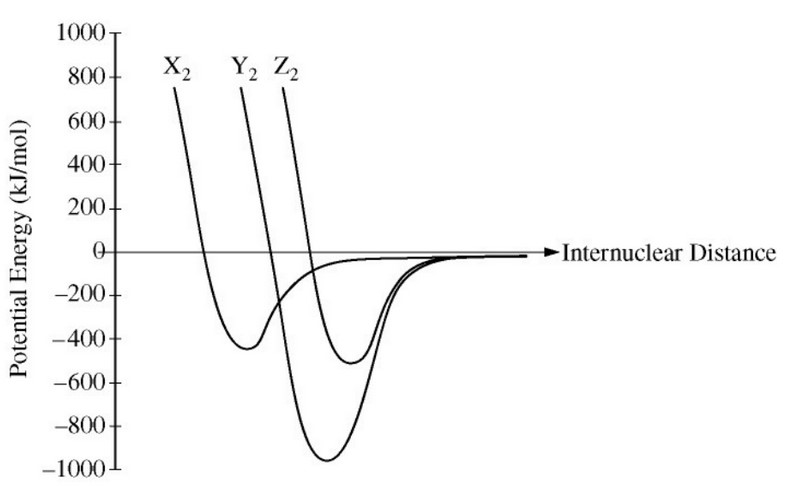
The potential energy as a function of internuclear distance for three diatomic molecules, \(\ce{X_2}\), \(\ce{Y_2}\), and \(\ce{Z_2}\), is shown in the graph above. Based on the data in the graph, which of the following correctly identifies the diatomic molecules, \(\ce{X_2}\), \(\ce{Y_2}\), and \(\ce{Z_2}\) ?
$$ \begin{array}{c c c c} & \ce{X2} & \ce{Y2} & \ce{Z2} \\ \hline \text{(A)} & \ce{H2} & \ce{N2} & \ce{O2} \\ \text{(B)} & \ce{H2} & \ce{O2} & \ce{N2} \\ \text{(C)} & \ce{N2} & \ce{O2} & \ce{H2} \\ \text{(D)} & \ce{O2} & \ce{H2} & \ce{N2} \\ \end{array} $$